As well as proving effective, it was also safe with no serious reactions linked to the vaccine during the trial.
Some side-effects to a vaccine are expected, but these are usually mild, including a sore arm, tiredness and a bit of a temperature. There were no deaths or serious illnesses in the vaccinated group linked to the jab.
Sputnik V has been approved so far in 60 countries, including Argentina, Palestinian territories, Venezuela, Hungary, UAE and Iran.
When will Sputnik V be available in India?
The Russian Direct Investment Fund (RDIF), which is marketing the vaccine, has signed deals to produce more than 750 million doses of Sputnik V in India with six domestic vaccine makers, according to reports.
Hyderabad-based pharmaceutical major Dr Reddy's Laboratories will be importing the first batch of 125 million doses to India during this quarter.
Supplies will be ramped up only next quarter when six Indian firms begin making the vaccine under the supervision of Dr Reddy's.
Until then, India will mostly depend on two previously approved candidates, Covaxin and Covishield.
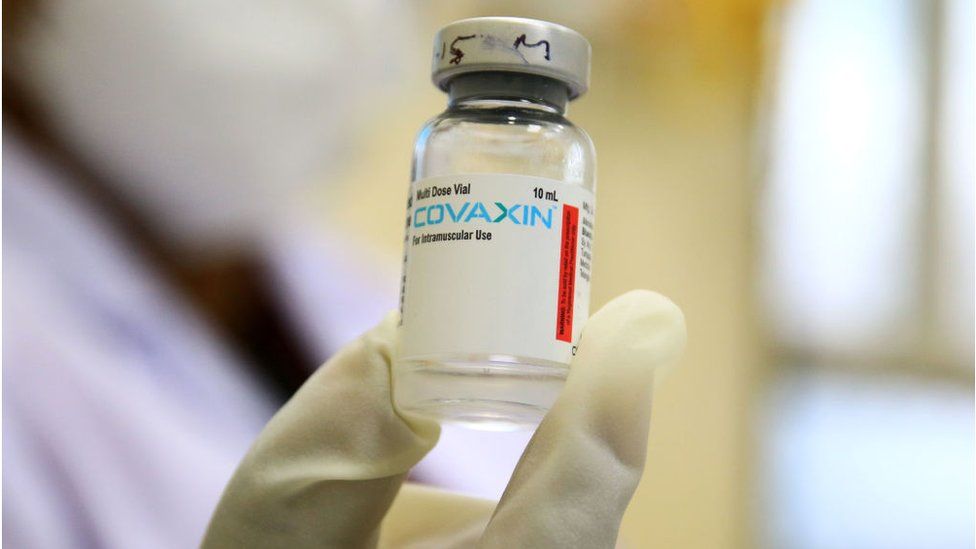
So what do we know about Covaxin?
Covaxin is an inactivated vaccine which means that it is made up of killed coronaviruses, making it safe to be injected into the body.
Bharat Biotech, a 24-year-old vaccine maker with a portfolio of 16 vaccines and exports to 123 countries, used a sample of the coronavirus, isolated by India's National Institute of Virology.
When administered, immune cells can still recognise the dead virus, prompting the immune system to make antibodies against the pandemic virus.
The two doses are given four weeks apart. The vaccine can be stored at 2C to 8C.
The vaccine has an efficacy rate of 81%, preliminary data from its phase 3 trial shows.
India's regulators gave the vaccine an emergency approval in January while the third phase of the trial was still underway, sparking scepticism and questions from experts.
Bharat Biotech says it has a stockpile of 20 million doses of Covaxin, and is aiming to make 700 million doses out of its four facilities in two cities by the end of the year.
What was the controversy around Covaxin?
It all began when the regulator in January said the vaccine had been approved for "restricted use in emergency situations in public interest as an abundant precaution, in clinical trial mode, especially in the context of infection by mutant strains".
Experts wondered how a vaccine was cleared for emergency use by millions of vulnerable people when its trials were still underway. The All India Drug Action Network at the time said that it was "baffled to understand the scientific logic" to approve "an incompletely studied vaccine". It said that there were "intense concerns arising from the absence of the efficacy data".
Both the manufacturer and drug regulator had defended Covaxin, saying it was "safe and provides a robust immune response".
Bharat Biotech had said that Indian clinical trial laws allowed "accelerated" authorisation for use of drugs after the second phase of trials for "unmet medical needs of serious and life-threatening diseases in the country". It had promised to provide efficacy data for the vaccine by February, which it has now done.
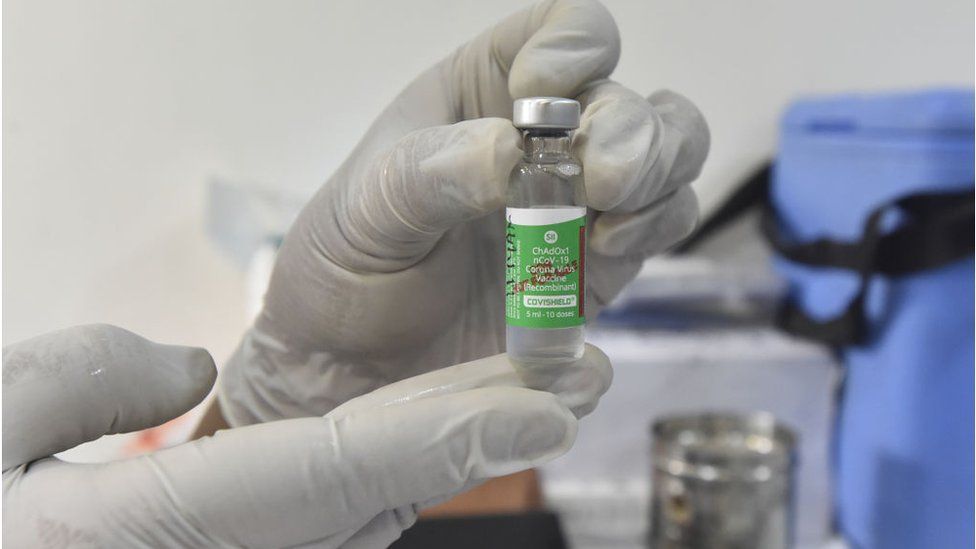
What about Covishield?
The Oxford-AstraZeneca vaccine is being manufactured locally by the Serum Institute of India, the world's largest vaccine manufacturer. It says it is producing more than 60 million doses a month.
The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus - although it can't cause illness.
When the vaccine is injected into a patient, it prompts the immune system to start making antibodies and primes it to attack any coronavirus infection.
The jab is administered in two doses given between four and 12 weeks apart. It can be safely stored at temperatures of 2C to 8C and can easily be delivered in existing health care settings such as doctors' surgeries.
The jab developed by Pfizer-BioNTech, which is currently being administered in several countries, must be stored at -70C and can only be moved a limited number of times - a particular challenge in India, where summer temperatures can reach 50C.
How effective is Covishield?
International clinical trials of the Oxford-AstraZeneca vaccine showed that when people were given a half dose and then a full dose, effectiveness hit 90%.
But there was not enough clear data to approve the half-dose, full-dose idea.
However, unpublished data suggests that leaving a longer gap between the first and second doses increases the overall effectiveness of the jab - in a sub-group given the vaccine this way it was found to be 70% effective after the first dose.
No comments:
Post a Comment